

Investigator Responsibilities
Investigator
Ensure subject safety
Develop reliable data
How investigator should be?
Have Good Resource
Investigators’ qualification
Investigator Qualifications
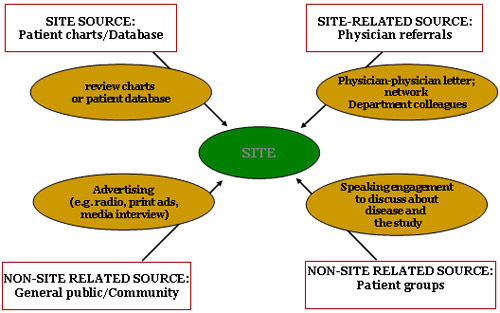
Recruitment Potential
Recruitment Strategy
Recruitment Source
Have Good Resource
Facility

Understand IRB Requirement
Understand Inform Consent Process
Execute Effective Subject Management
Adhere to Protocol
Werayut Kunasirirat
Aug 11, 2010
Aug 11, 2010
Investigator
Ensure subject safety
Develop reliable data
How investigator should be?
- Have good resource
- Understand IRB requirement
- Understand inform consent process
- Execute effective patient management
- Adhere to Protocol
Have Good Resource
Investigators’ qualification
- Education
- Training
- Experience
Investigator Qualifications
- Understand and Comply with GCP
- Familiar with study product
- Understand Study protocol
Recruitment Potential
- Subject pool
- Subject resource
Recruitment Strategy
- Study design and Timeline
- Subject Population (healthy subject or patient)
- Aware of study challenge and timeline
- Recruitment Source
- Recruitment Method
- Recruitment Material
- Recruitment Plan
- Recruitment Tracking
Recruitment Source

Have Good Resource
- Qualified staff
- Qualified facilities
Facility

- Investigator has sufficient time to conduct and complete the study
- Site staffs are adequately informed on study related information.
Study Product
|
 |
- inventory - Use of study product
|
 |
Understand IRB Requirement
- Provide study related doc to IRB/ERC for review and approve
- Protocol, Inform consent, investigator’s brochure or others - Sign protocol or alternative contract to confirm an agreement
- Ensure the written approval on protocol, inform consent and other written doc provided to subject
- Provide any updated doc for review and approval
- Protocol, Inform consent, investigator’s brochure or others - Progress Report
- Submit study update to IRB/ERC accordingly
- Promptly report to IRB/ERC if any significant change to study or increase risk to subject - Safety Report
- Immediately report any serious adverse event to sponsor
- Follow IRB/ERC regulation on any of AE report
- Use code number to identify subject
- In case of dead, additional information such as autopsy is needed - Final Report
- Notify IRB/ERC upon completion
- Providing IRB/IEC with a summary of the trial’s outcome
Understand Inform Consent Process
- Obtain IC before a subject enters the study
- Use the most understandable language to communicate
- Avoid coerce or unduly influence a subject
- No language waive or appear to waive any legal right or liability for negligence
- Provide time and opportunity to read and decide
- Answer all questions
|
 |
- An impartial witness is presented through out the process of inform consent if a subject is unable to read
- An impartial witness must personally sign and date
- New information in inform consent should be informed to a subject in the timely manner after IRB/ERC review and approve
Execute Effective Subject Management
- Medical Care of Subject
- Responsible for study related medical decisions
- Adequate medical care is provided in the event of AE during the study
- Inform a subject’s primary doctor of subject participation - Premature Termination/Suspension
- Promptly inform subject
- Assure appropriate therapy and follow up for the subject
- Comply with any IRB/ERC or regulatory requirement
- Sponsor
- IRB/IEC
- Provide written explanation to sponsor and IRB/IEC for the termination
- Inform the institution
- Inform IRB/IEC and Investigator
- provide explanation of the termination or suspension
Adhere to Protocol
- Randomization procedures and unblinding
- Following protocol randomization procedure
- Unblinding according to protocol
- Document and notify on any premature unblinding - Record and Report
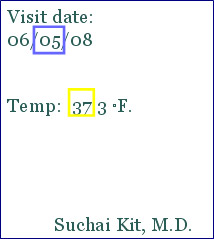
- Ensure accuracy, completeness, legibility and timeliness of data entered to the case report form
- Ensure the consistency of the data
- Ensure that any change to the data is initialed, dated and explained
Example:
| Source Document | CRF |
 |
 |
- Retain any document drop under essential document
- Prevent accidental or premature destruction of document
- Make available for monitoring , auditing or inspecting
| Essential Document (ICH GCP 8) |