

Introduction to Drug Development Process
Objectives
- Recognize the investigational drug success rates by stages
- Define pre-clinical studies
- Define Investigational New Drug Application Phase I, II, III Studies
- Define New Drug Application
- Define phase IV studies
Investigational Drug Success Rates by Stage
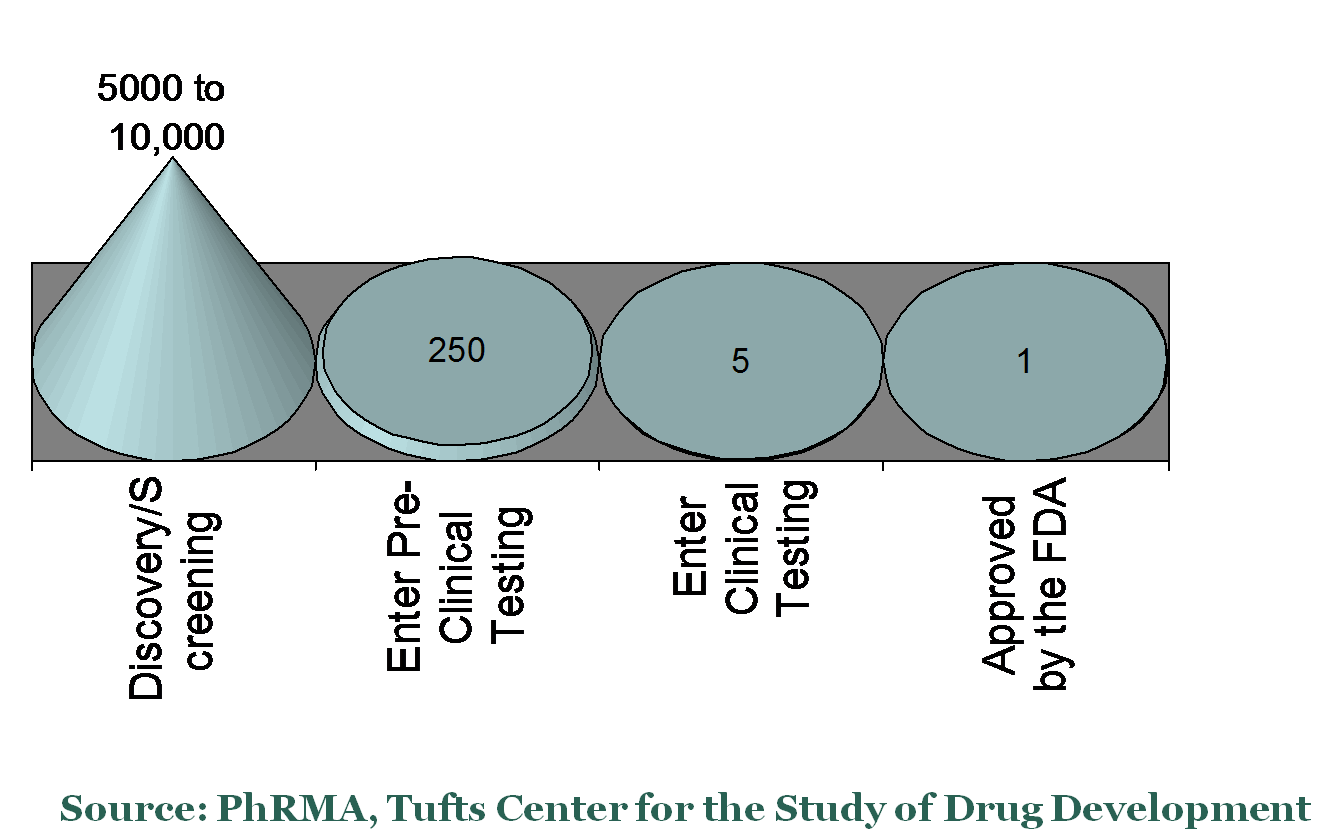
.png)
Pre-Clinical Studies
- Single Dose Toxicity Studies
- Repeated Dose Studies
- Safety Pharmacology Studies
- Genotoxicity Studies
- Carcinogenicity Studies
- Reproductive Toxicity Studies
- Data Collected in Animal Studies
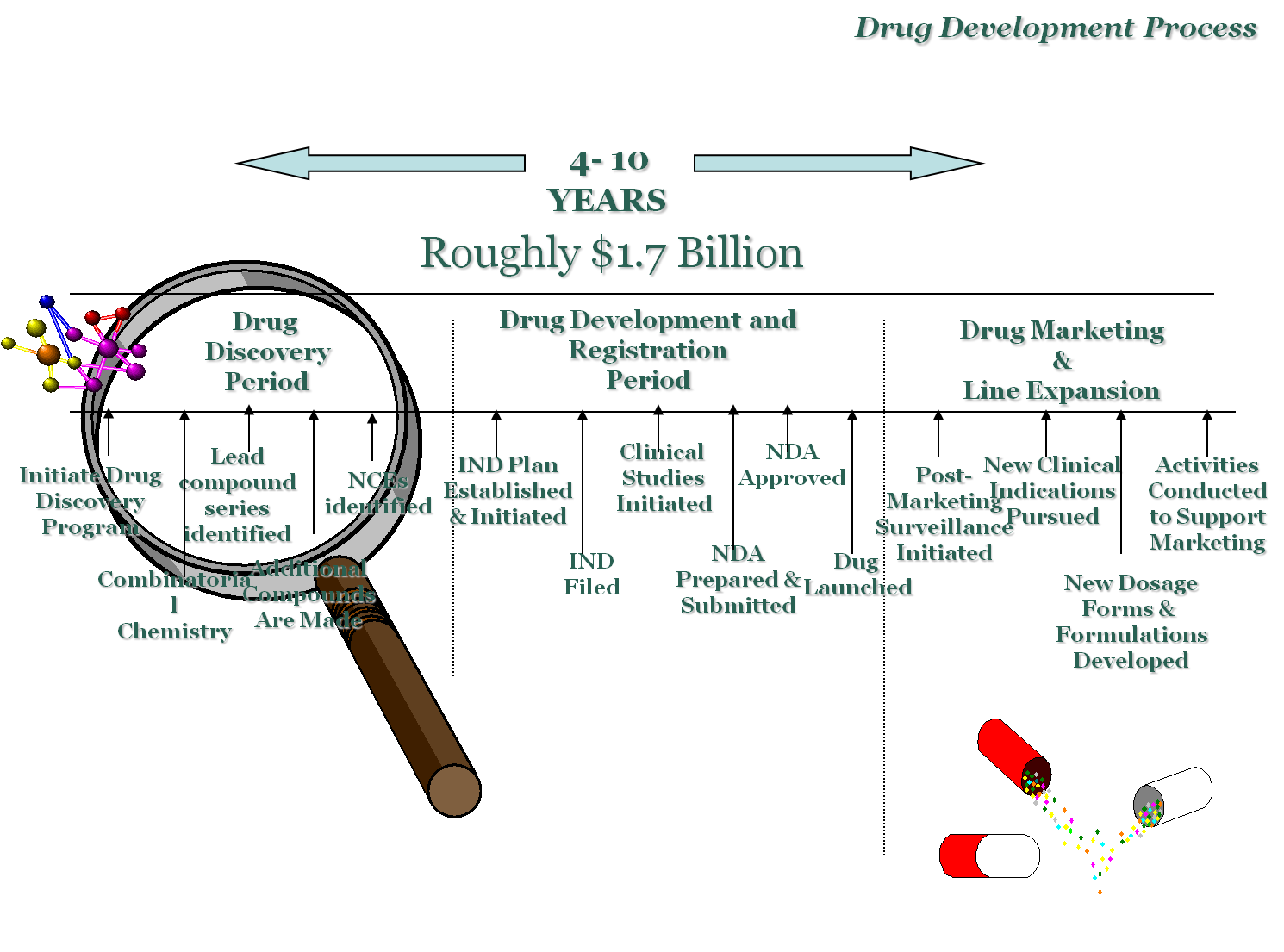
Investigational New Drug Application
The product of successful Preclinical development
If the investigational New Drug submission is accepted, the compound begins Phase I –IV clinical trials. Long-term preclinical trials continue.
Clinical Research
Phase I : First in Humans
Trial Design
- Normal, usually healthy, volunteer subjects
- Few subjects (20-100)
- Typically single center
- Usually no benefit to subjects
- Duration: Short – from days to several weeks or months
- Open label – no placebo or comparative agent
- Uncontrolled
- Single or multiple doses